17 Feb What are electrons and why are they important
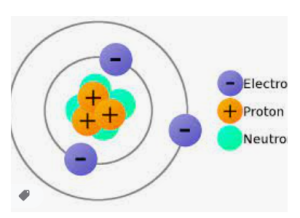
Everything on earth is made up of atoms and these atoms contain both electrons and protons. It is the number of these particles that make up the characteristics of the element and also determine its position on the periodic table. Copper, for example, is used to make 6mm Copper Pipe that you can get from https://watkinspowis.co.uk/products/copper-pipe-fittings-and-press-systems and it carries an atomic number of 29. This means that there are 20 protons and electrons in its atom.
Electrons are the negatively charged particles that are found in the atom of any element. The number of negatively charged electrons is equal to the positive protons that are also found in the atom. Electrons are incredibly small and move at incredibly high speeds. It is for this reason that it is impossible to pinpoint where the electrons are at any given moment in time.
As well as forming a part of every atom, electrons also play an important role in chemical bonds. This bonding occurs in one of two ways – either electrovalent which is where an electron from one atom is transferred across to another atom, and covalent which is where an electron is shared between the two atoms. The kind of bonding that takes place will depend on the atoms being joined as well as the requirement for the final item.